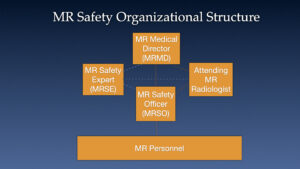
Guest Karl Vigen, PhD joins Bill, Kristan and Howard in a wide ranging discussion of MR safety considerations in patient with implants, devices or unknown metallic items.
The content of this Contrast Enhanced MRI Program is intended for healthcare professionals who work in the MRI environment. These individuals include MRI Technologists, Imaging Nurses, MRI Researchers, and Others.
This program has been submitted for 1.00 hours of Category A CE credit as designated by the International Society for MR Radiographers & Technologists (ISMRT) RCEEM.
Release Date: 06/23/2024 | Expires: 06/22/2025
Faculty and Planner Disclosures: In accordance with this policy, faculty and planners must disclose any financial relationships with commercial interests germane to program content. Additionally, in the event a conflict of interest (COI) does exist, it is the policy of NWIF to ensure that the COI is resolved in order to ensure the integrity of the CME/CE activity. The planner has nothing to disclose